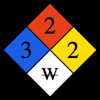
Notes on the properties of Lithium: Critical Pressure: Value estimated based on extrapolation. Critical Temperature: Value estimated based on extrapolation. Specific Heat: Value given for solid phase. Up to date, curated data provided by Mathematica's ElementData function from Wolfram Research, Inc. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||